Introduction
The concept of bioeconomy has gained global attention as a means of promoting the use of renewable biological resources to produce goods and services across various sectors, including agriculture, healthcare, industry, and energy. It spans from traditional utilization of non-timber forest products—such as vegetable oils, fruits, seeds, and resins—to high-tech applications including pharmaceuticals, cosmetics, and green chemistry.
In Brazil, bioeconomy has gained relevance in the Amazon, often presented as a new paradigm that aligns biodiversity conservation with sustainable resource use, while valuing traditional knowledge and strengthening production models based on biological resources (Lopes and Chiavari 2022). From the perspective of a “sociobiodiversity bioeconomy,” many actors advocate for agroforestry systems, the utilization of non-timber forest products, and community-based solutions to promote sustainable development in the region (Euler, Aubertin and Cialdella 2023; Uma Concertação pela Amazônia 2023; Feltran-Barbieri et al. 2025). On the other hand, a more innovation-oriented approach focused on biotechnology and commercialization of biodiversity-based products has been less explored.
With its immense biological and cultural diversity, the Amazon has significant potential to develop value chains linked to biodiversity. Biodiversity-based biotechnology can add value by generating products of higher quality and with greater market value. However, this approach remains constrained by regulatory, institutional, financial, and infrastructure challenges.
This study, conducted by researchers from Climate Policy Initiative/Pontifical Catholic University of Rio de Janeiro (CPI/PUC-RIO) and the Amazon 2030 project, presents a regulatory and institutional mapping of biodiversity-based biotechnology examined through the intersection of three dimensions—biotechnology, biodiversity, and bioeconomy—and identifies the main legal frameworks and institutions involved in this agenda in Brazil. It then deepens the analysis of regulatory and institutional challenges, focusing on the Access and Benefit-Sharing (ABS) framework that governs access to genetic resources and associated traditional knowledge and establishes benefit-sharing rules, while also highlighting specific impacts in the Amazon.
The study concludes that, although Brazil’s bioeconomy is advancing on multiple fronts, biodiversity-based biotechnology is still far from reaching its potential to generate economic value, support conservation, and strengthen local value chains, especially in the Amazon. Overcoming regulatory hurdles and enhancing institutional coordination can create a more predictable and enabling environment for research and business. In this regard, the forthcoming National Bioeconomy Development Plan (Plano Nacional de Desenvolvimento da Bioeconomia – PNDBIO) represents a strategic opportunity to explicitly recognize biodiversity-based biotechnology and integrate it as a central component of Brazil’s bioeconomy strategy.
Key Messages
Challenges associated with Brazil’s Access and Benefit-Sharing (ABS) Framework
• Amid multiple regulatory layers, biodiversity regulation brings the greatest challenges
Biodiversity-based biotechnology is subject to regulatory frameworks in three dimensions: biotechnology, biodiversity, and bioeconomy. While biotechnology inherently falls under multiple sectoral regimes, the biodiversity regulation poses the greatest complexities and challenges in terms of governance, legal certainty, and implementation. These challenges restrict the potential of biodiversity-based biotechnology to enhance sociobiodiversity value chains in the Amazon, diversify local economies, create new markets, generate jobs, and reduce pressures on forests.
• Benefit-sharing falls short of expectations
Although the mechanism established under the Legal Framework on Access and Benefit-sharing was conceived with the promise of generating resources for Indigenous Peoples and Local Communities (IPLCs) and supporting biodiversity conservation, the results are far below the expectations. Despite this, the National System for the Management of Genetic Resources and Associated Traditional Knowledge (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado – SISGEN) data show that in the Amazon, where Indigenous peoples and traditional communities are strongly present, a significant share of biodiversity-based products involves associated traditional knowledge of identifiable origin, requiring direct benefit-sharing with local communities. However, legal exemptions in benefit-sharing, a concentration of benefit-sharing in a few sectors, legal uncertainties, delays in approving benefit-sharing agreements, and poor operationalization of the National Benefit-Sharing Fund (Fundo Nacional para a Repartição de Benefícios – FNRB) have limited the effective flow of resources. For these reasons, biodiversity-based biotechnology is unlikely to generate significant revenues through benefit-sharing, although it can strengthen sociobiodiversity value chains.
• Regulatory challenges discourage innovation and create legal uncertainty
The ABS framework has proven to be more of a barrier than an incentive to the development of biodiversity-based biotechnology in Brazil. The complexity of the SISGEN, the delays and uncertainty in approving benefit-sharing agreements, the risks introduced by the new regulation on reference lists of traditional knowledge, and the lack of clarity on how to address Digital Sequence Information (DSI) create a burdensome and unpredictable regulatory environment. These challenges affect researchers, startups, small businesses, and biodiversity-intensive sectors such as cosmetics, personal care, perfumery, and pharmaceuticals, which face significant barriers in transforming scientific knowledge into innovation and competing globally.
• Excessive requirements undermine investment, trade, and international cooperation
Brazil’s ABS framework imposes additional hurdles for foreign companies using Brazilian biodiversity-related resources and for domestic companies operating in international markets. Foreign firms must designate a representative under Brazilian jurisdiction and cannot rely on the country’s integration into the Nagoia Protocol’s ABS Clearing-House, making it more difficult for foreign countries to verify compliance. Additionally, the requirement for Material Transfer Agreements (MTAs) can delay or even prevent the deposit of samples and the publication of new species, thereby undermining scientific collaboration and product development abroad based on Brazilian genetic material. This combination of barriers discourages investment, fosters jurisdiction shopping—where companies shift activities to countries with simpler, more predictable, or even nonexistent rules—and risks reducing Brazil’s share in global biodiversity-based biotechnology markets.
Institutional Challenges and Opportunities for Biodiversity-Based Biotechnology
• Biodiversity-based biotechnology operates within a fragmented institutional environment
Biodiversity-based biotechnology has applications in sectors that fall under the responsibility of different national ministries, each with distinct strategies, policies, programs, objectives, and investments that do not always converge. For biodiversity-based biotechnology, this means operating in a dispersed institutional environment with limited coordination and unclear priorities, which undermines its recognition as a transversal strategic agenda.
• PNDBIO provides a strategic opportunity to include biodiversity-based biotechnology in the national agenda
The formulation of the PNDBIO represents a strategic moment to recognize the value of biodiversity-based biotechnology within Brazil’s bioeconomy. For this to occur, ministerial perspectives must first be aligned, and the plan’s current scope, limited to traditional sectors such as biomass, must be expanded to explicitly include biodiversity-based biotechnology as a central and strategic dimension.
Contextualizing Biodiversity-Based Biotechnology
Biotechnology can be understood as the use of living organisms—plants, animals, microorganisms, and their components—to develop products and processes with applications across multiple sectors of the economy. According to the Convention on Biological Diversity (CBD), biotechnology refers to “any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use” (MMA 2000). This definition encompasses traditional practices such as fermentation for food and beverage production, as well as modern technologies including genetic engineering, and cell culture, for the production of agricultural biologicals, biofuels, and innovative medicines (Gupta et al. 2017; Hilgartner 2015).
In relation to biodiversity, biotechnology uses elements of Brazil’s flora, fauna, and microbiota in research and innovation processes, shaping what this report refers to as biodiversity-based biotechnology. This approach expands opportunities for valuing Brazil’s genetic resources and associated traditional knowledge, generating high-value goods and services in areas such as health, agriculture, energy, cosmetics, and ecosystem restoration (Uma Concertação pela Amazônia 2024).
The development of Captopril, a drug used worldwide to control high blood pressure that is derived from the venom of the jararaca snake (Instituto Butantan 2023), and Acheflan, an anti-inflammatory developed from the shrub Cordia verbenacea, known in Brazil as erva-baleeira (ABIFINA 2015), illustrates how Brazil’s biodiversity can give rise to innovations with global impacts. Agricultural biologicals based on native microorganisms are also being used to control pests and diseases, replacing chemical pesticides at scale. In the energy sector, species such as the macaúba palm are being studied as biomass sources for biofuels (Machado et al. 2025). The economic potential of these innovations has been quantified: the Brazilian Bioinnovation Association (Associação Brasileira de Bioinovação – ABBI) estimates that an investment of US$ 257 billion in multiple bioeconomy-related technologies could generate a return of US$ 593 billion by 2050 (ABBI 2024).
In the Amazon, this potential becomes even more strategic as it links to sociobiodiversity value chains. Açaí, for example, traditionally commercialized as pulp, is now used through biotechnological applications that enable the production of supplements, natural dyes, and antioxidants (Alavarsa-Cascales et al. 2022). Oils and extracts are also used in cosmetics (Stehlgens, Silva and Carvalho 2024), while residues such as seeds are being studied for use in bioenergy and biosurfactants (Gibson 2024). Other natural ingredients, such as fruits, nuts, fibers, and resins, are also gaining new applications by Brazilian institutions such as the Brazilian Agricultural Research Corporation (Empresa Brasileira de Pesquisa Agropecuária – EMBRAPA), the Amazon BioBusiness Center (Centro de Bionegócios da Amazônia – CBA), and the Vale Institute of Technology (Instituto Tecnológico Vale – ITV). This diversification strengthens sociobiodiversity value chains, increases income generation, and promotes circular economy practices that fully utilize native species. Emerging fields, such as forest restoration, can also benefit from biotechnological processes applied to soil ecology, seedling development, and the selection of carbon-fixing microorganisms (Peddle et al. 2025).
By adding value to biodiversity, fostering innovation across productive sectors, and linking environmental, social, and economic dimensions, biodiversity-based biotechnology stands out as a transversal and strategic agenda. It bridges science, conservation, and sustainable development, but its consolidation depends on integrated policies that can align regulation, finance, and innovation. The next section presents a regulatory and institutional mapping of biodiversity-based biotechnology in Brazil, identifying the relevant legal frameworks and institutions involved in this agenda.
Regulatory and Institutional Mapping of Biodiversity-Based Biotechnology
Regulatory Mapping
Biotechnology is inherently cross-cutting, with applications across multiple sectors. It spans from scientific research to technological innovation, and is therefore directly connected to legal frameworks on science, technology, and innovation designed to foster research and development (R&D). It also drives the work of companies and startups that transform scientific knowledge into innovative solutions within an ecosystem where intellectual property plays a critical role in securing discoveries. Depending on the sector, biotechnology is also subject to environmental, health, or biosafety regulations. By its very nature, then, biotechnology operates within a broad regulatory landscape.
When applied to Brazilian biodiversity, however, biotechnology is subject to a specific regime: the Access and Benefit-Sharing (ABS) framework, which comprises international treaties and national legislation, particularly Law no. 13,123/2015, its implementing decree, and complementary norms established by the Genetic Heritage Management Council (Conselho de Gestão do Patrimônio Genético – CGEN). This framework introduces specific obligations that make the use of biodiversity in biotechnology more complex than in other sectors.
In contrast, bioeconomy has been presented as an integrative dimension, capable of bridging biodiversity and biotechnology. The National Bioeconomy Strategy (Estratégia Nacional de Bioeconomia – ENBIO) reflects this perspective by linking the sustainable use of biodiversity to scientific and technological innovation, aiming to align conservation, value creation, and inclusive development.
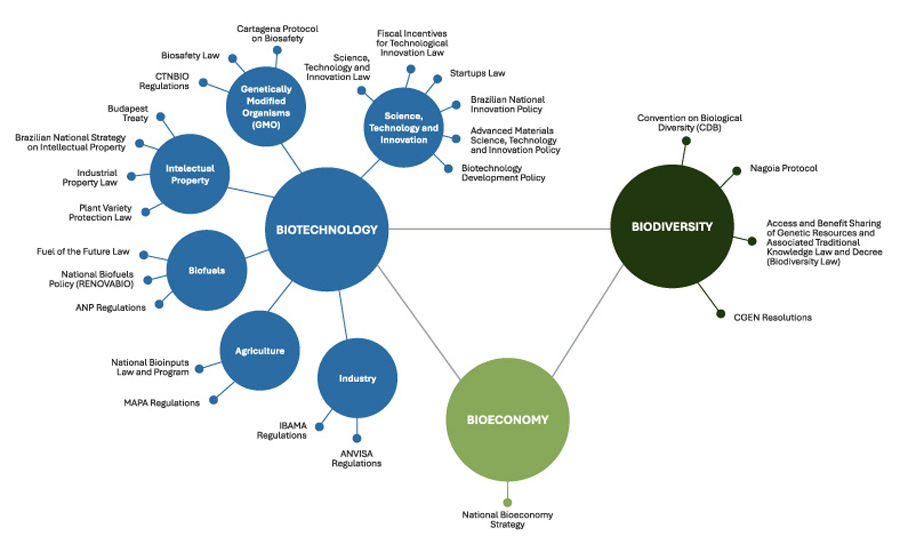
Figure 1 below presents the main legal frameworks associated with each dimension of biodiversity-based biotechnology.
Figure 1. Regulatory Mapping of Biodiversity-Based Biotechnology
Source: CPI/PUC-RIO, 2025
This mapping highlights that biotechnology is subject to a wide and fragmented set of regulations across multiple sectors. Yet, when applied to biodiversity, it faces the specific ABS framework that introduces additional obligations and complexity. While biotechnology is governed by a larger number of legal instruments, it is biodiversity regulation that imposes the most significant challenges in terms of governance, legal certainty, and implementation. This asymmetry helps explain why bioeconomy is often portrayed as an integrative dimension that aims to align innovation, conservation, and development under a single strategic horizon, even if its effectiveness depends on governance and practical implementation.
Institutional Mapping
Biodiversity-based biotechnology has applications in sectors that fall under the responsibility of different national ministries, although it is not explicitly addressed by any of them. Biotechnology is reflected primarily in bioeconomy programs.
- The Ministry of Environment and Climate Change (Ministério do Meio Ambiente e Mudança do Clima – MMA) leads initiatives related to genetic resources and biodiversity conservation through its National Secretariat of Bioeconomy, its presidency of the CGEN, and its management of the SISGEN.
- The Ministry of Development, Industry, Trade and Services (Ministério do Desenvolvimento, Indústria, Comércio e Serviços – MDIC), which currently presides over the National Bioeconomy Commission (Comissão Nacional de Bioeconomia – CNBIO), works on bioindustry policy and executes the New Industry Brazil strategy through its Secretariat of Industrial Development, Innovation, Trade and Services.
- The Ministry of Science, Technology, and Innovation (Ministério da Ciência, Tecnologia e Inovação – MCTI) plays a strategic role in supporting research and innovation. It coordinates the National Technical Commission on Biosafety (Comissão Técnica Nacional de Biossegurança – CTNBIO), responsible for genetically modified organism (GMO) policies, and promotes bioeconomy programs through its Secretariat for Strategic Policies and Programs.
- The Ministry of Agriculture and Livestock (Ministério da Agricultura e Pecuária – MAPA) defines guidelines for bio-inputs and biomass production, with EMBRAPA as the main research institution in agricultural biotechnology.
- The Ministry of Mines and Energy (Ministério de Minas e Energia – MME) contributes to the biofuels agenda through its National Secretariat of Oil, Natural Gas, and Biofuels, which oversees the implementation of the Fuel of the Future Law.
This institutional diversity results in different—and sometimes divergent—visions and priorities for bioeconomy. While the MMA emphasizes sociobiodiversity and the sustainable use of natural resources, ministries such as the MDIC and MAPA focus on bioindustry and biomass, respectively.
This heterogeneity is more programmatic than conceptual: it is expressed in strategies, policies, programs, and investments that do not always converge or directly address biodiversity. For biodiversity-based biotechnology, this translates into a fragmented institutional environment, with limited coordination and unclear priorities, which hinders its recognition as a transversal strategic agenda.
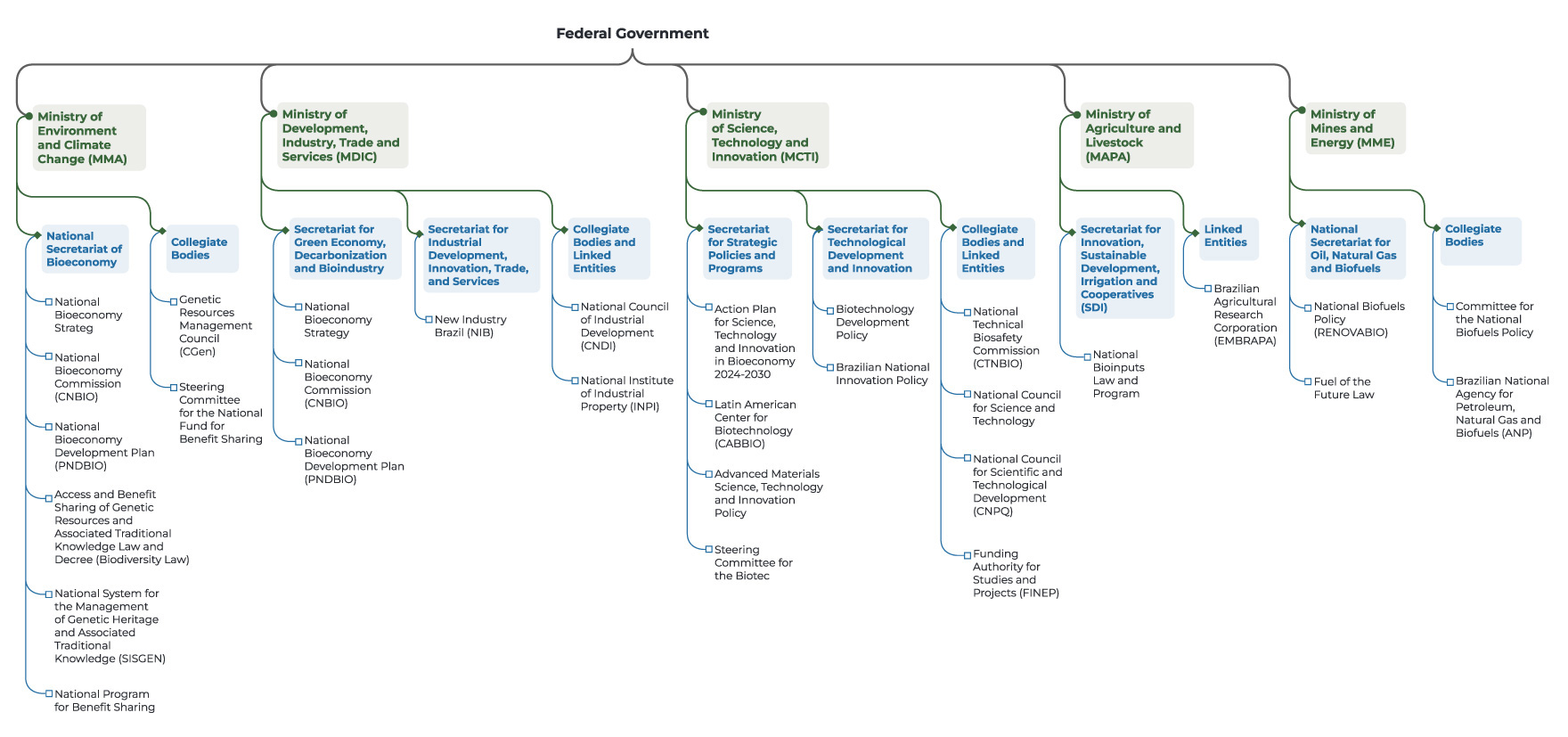
Figure 2 presents the main ministries and their secretariats, as well as the collegial bodies, committees, and related programs connected to biotechnology, biodiversity, and bioeconomy. While not exhaustive, it brings together the most relevant institutions and policies to illustrate the institutional structure of this agenda in Brazil.
Figure 2. Institutional Mapping of Biodiversity-Based Biotechnology
Source: CPI/PUC-RIO, 2025
Regulatory and Institutional Challenges for Biodiversity-Based Biotechnology
The Access and Benefit-Sharing Framework
Brazil’s national ABS framework is established by Law no. 13,123/2015, its implementing decree (no. 8,772/2016), and complementary regulations issued by the CGEN. Together, these rules define the conditions for access (research and/or technological development) related to genetic resources and associated traditional knowledge, as well as the obligations for benefit-sharing.
The law sets out the following core definitions[1]
Access: Research and/or development involving genetic resources and/or associated traditional knowledge.
Genetic resources: Genetic information (DNA/RNA) contained in plants, animals, microorganisms, or other living organisms, including substances derived from their metabolism, such as proteins, enzymes and essential oils.
Associated traditional knowledge: Knowledge and practices of IPLCs, and traditional farmers associated with the use of genetic resources, which may be of identifiable origin (when it is possible to identify at least one community that holds the knowledge) or non-identifiable origin (when no specific people or community can be identified as the source of such knowledge).
Holder: Whoever holds traditional knowledge.
Provider: Whoever grants access to their traditional knowledge.
User: Person or institution that carries out access or develops products with genetic resources and/or associated traditional knowledge.
Finished product: A product ready for use by the final consumer in which genetic resources or associated traditional knowledge add value, such as shampoo or medicine made with extracts from native plants.
Intermediate product: Input (excipients and raw materials) used along the production chain to manufacture other products.
Reproductive material: Such as seeds, seedlings, cuttings, parts of stems, branches, or roots used to generate new plants, for instance.
The law establishes two central obligations: (i) registration of access in the SISGEN whenever research or development involves genetic resources and/or traditional knowledge; and (ii) benefit-sharing, which may be monetary or non-monetary. Registration is required before taking strategic steps, such as publishing research results, shipping samples abroad for research or development, filing patents, or commercializing intermediate products. For finished products and reproductive material, both registration and notification in SISGEN are required prior to commercialization to ensure benefit-sharing is implemented.
Law no. 13,123/2015, which replaced Provisional Measure no. 2,186-16/2001, imposed heavy bureaucracy and drove many research institutions and companies into illegality (Malavazi et al. 2025). While the new regime simplified access to genetic resources, challenges remain—notably the complexity of SISGEN and difficulties in interpreting legal obligations (MDIC, MF and MMA 2025; Malavazi et al. 2025; Maia and Bourgeois-Gironde 2025; ICC Brasil 2025; Jungman and Avila 2022; Farias, Maia, and Lima 2022; Instituto Escolhas 2021; CGEE 2020; CNI 2020; Bockmann et al. 2018; Fiocruz 2018). Moreover, Brazil’s experience mirrors a broader global issue: ABS frameworks adopted by different countries have so far failed to generate significant resources for biodiversity conservation or deliver tangible benefits to IPL Cs (Maia and Bourgeois-Gironde 2025; The Economist 2025).
New CGEN Resolution and the Creation of Reference Lists of Associated Traditional Knowledge
CGEN Resolution no. 47, published on August 27, 2025, establishes the creation of reference lists of associated traditional knowledge, distinguishing between knowledge of identifiable origin—with a clear indication of its providers—and that of non-identifiable origin. These lists will be prepared based on information such as the description of the associated traditional knowledge, the scientific and common names of Brazilian biodiversity species, the biome of occurrence, and identified providers. These lists can be updated at any time upon request from IPLCs. The resolution also introduces a voluntary consultation procedure during SISGEN registration to verify whether providers linked to the accessed knowledge exist.
This measure aligns with broader proposals to create traditional knowledge databases, based on the assumption that all research and development draws on preexisting traditional knowledge and seeks to expand benefit-sharing with IPLCs (Instituto Escolhas 2023; Uma Concertação pela Amazônia 2024). However, it is important to recall that legislative and administrative measures directly affecting these groups must comply with Article 6 of the International Labour Organization’s (ILO) Convention 169, which requires the free, prior, and informed consent of communities.
In principle, the list could enhance legal certainty for users in cases where the holders of traditional knowledge are unclear. By offering an official reference, it could help identify providers in situations of uncertainty and reduce the risk of disputes over the legitimacy of benefit-sharing agreements already concluded. In such cases, the lists would function as an institutional safeguard for obtaining prior informed consent and formalizing benefit-sharing, thereby reducing the exposure of researchers and companies to future challenges.
At the same time, the resolution creates significant risks for both communities and users (companies and researchers). It may exclude communities and peoples lacking the capacity to initiate the administrative process required for inclusion as knowledge holders, favor competition among providers, and concentrate benefits in more organized communities, reinforcing inequalities. In the scientific field, the consequences may be even more severe: if genetic resources are linked to identifiable traditional knowledge, conducting research would necessarily require obtaining prior informed consent. In practice, this requirement could affect a large share of applied biodiversity research in Brazil, lengthening timelines, increasing bureaucratic steps, and creating uncertainty over the viability of ongoing and future projects.
Another challenge is the absence of deadlines for CGEN’s plenary to conclude consultations requested by users, which could indefinitely delay registrations and notifications and even block product commercialization.
Finally, by assuming that listed traditional knowledge is inherently tied to genetic resources, the resolution expands the concept of access beyond what is established by Law no. 13,123/2015, which requires an effective link between knowledge and access to genetic resources. In other words, access to traditional knowledge only occurs when research or product development makes use of such knowledge, whether through direct interaction with communities or through secondary sources such as scientific articles, fairs, and books.
National System for the Management of Genetic Resources and Associated Traditional Knowledge
SISGEN is Brazil’s electronic platform where all registrations of access and notifications of finished products and reproductive material must be recorded. Its primary function is to allow public authorities to monitor the use of genetic resources and associated traditional knowledge, ensuring traceability across each stage of R&D, from research to the commercialization of products subject to benefit-sharing, and thereby securing compliance with the ABS framework.
From its inception, SISGEN has faced operational challenges (ABIFINA 2017). Following its launch, several CGEN resolutions were issued to adjust the system and provide solutions for situations where it did not accurately reflect the realities of scientific research (MMA 2025a). To this day, the interface remains non-intuitive and lacks guidance tools. Requirements such as exact geographic coordinates of collection sites and detailed third-party data make the system cumbersome and user-unfriendly (Instituto Escolhas 2021; Malavazi et al. 2025).
Large research institutions and companies usually manage to comply with SISGEN obligations by relying on dedicated teams or consultants. Independent researchers, startups, and small or medium-sized enterprises, however, often lack the resources or expertise to navigate the system. Without adequate support, there is a high risk of errors in data entry.
Like any system that evolves through use, SISGEN requires continuous updates and improvements. Yet, maintenance has been insufficient to meet users’ needs, particularly foreign users. For years, the system has been awaiting a new version that would enable foreign institutions to operate the system directly. In June 2025, the Department of Genetic Heritage(Departamento de Patrimônio Genético – DPG) launched a testing SISGEN module for non-Brazilian institutions.[2] This module enables institutions and individuals based outside Brazil to simulate the creation of an account for foreign users and institutions, as well as carry out registration in association with Brazilian partners, and notification of finished products and reproductive materials. The purpose of this pilot phase is to collect feedback before the official release of the new SISGEN version, expected later in 2025 (Souto Correa Advogados 2025).
The real problem with SISGEN is not users’ willingness to comply, but the complexity of the system itself. The burdens created by its interface and data requirements generate compliance difficulties, particularly for smaller actors.
Benefit-Sharing
Manufacturers of finished products or reproductive materials that use genetic resources or associated traditional knowledge are required to share part of their revenues with the holders of these resources. The rules are not the same for genetic resources and traditional knowledge.
For genetic resources, users may choose between two options: depositing 1% of the net revenue from sales of finished products or reproductive material into the FNRB (monetary benefit-sharing), or entering into a benefit-sharing agreement with the federal government (non-monetary benefit-sharing). Under the latter option, between 0.75% and 1% of revenues must be allocated to initiatives such as conservation projects or capacity-building for local communities.
The rules for associated traditional knowledge vary depending on whether the knowledge is of identifiable or non-identifiable origin. When knowledge is identifiable, the user must negotiate directly with the community that holds and provides it, while also depositing 0.5% of revenues into the National Benefit-Sharing Fund to ensure that other communities that hold the same knowledge also receive a share. If the knowledge is of non-identifiable origin, the user must deposit 1% of net revenues into the Fund.
However, a series of exemptions reduces the scope of benefit-sharing obligations.
Intermediate products used as inputs, excipients or raw materials in the production chain are exempt from benefit-sharing. For example, a processed ingredient—such as a plant extract, an enzyme, or a technological formulation (like encapsulated ingredients for controlled release)—or even a finished product itself, such as an essence/essential oil or a herbal medicine, which will then be incorporated into the formulation of a final product, such as a perfume or a dietary supplement. This exemption is particularly significant regarding biodiversity-based biotechnology, since many ingredients used in food, cosmetics, personal care, perfumery, and pharmaceuticals are produced by intermediary companies. In these cases, the responsibility for benefit-sharing falls on the manufacturers of finished products, concentrating obligations on actors with greater financial capacity.
Agricultural inputs, such as biofertilizers for plant nutrition, biostimulants for growth, and biological control agents for pests and diseases, are also classified as intermediate products and are exempt from benefit-sharing. This rule benefits startups and medium-sized firms working with biologicals, but it also exempts large agrochemical companies.
Another exemption applies when renewable inputs replace fossil raw materials. For instance, when a Brazilian microorganism is used to produce a substance chemically identical to one derived from petroleum, no benefit-sharing is required. One example is “green plastics”: Sugarcane is fermented with microorganisms to produce ethanol, which is then transformed into ethylene—the same molecule obtained through petroleum refining. Ethylene, whether of biological or fossil origin, is used to produce polyethylene, one of the most widely used plastics in the world. In other words, although the production pathway differs (biological rather than fossil), the resulting raw material (ethylene) is exactly the same, which is what justifies the exemption. This exception has a major impact: by exempting benefit-sharing when fossil raw materials are replaced with renewable alternatives, the legislation encourages the transition to a low-carbon economy. At the same time, however, it reduces the potential for channeling resources to biodiversity conservation, even when bioeconomy seeks to add value to biodiversity through biotechnology.
Micro and small enterprises, individual entrepreneurs, traditional farmers, and their cooperatives are also exempt from benefit-sharing obligations. This measure acknowledges that these actors operate with lower margins and limited financial capacity compared to large companies.
In practice, Brazil’s ABS framework concentrates benefit-sharing obligations mainly on industries producing finished products, particularly in the cosmetics, personal care, perfumery, and pharmaceuticals sectors. As a result, the overall reach and impact of resources destined for IPLCs, and biodiversity conservation initiatives, remain limited.
SISGEN data illustrates how benefit-sharing occurs in practice, while also highlighting the main challenges of the mechanism (see Box 1).
Box 1. SISGEN Data on Products Subject to Benefit-Sharing
SISGEN has a public module that displays notifications of finished products that are subject to benefit-sharing. Although the system has limitations, particularly for structured data extraction, it is possible to access relevant information such as the modality of benefit-sharing (monetary or non-monetary), whether the access involves genetic resources or traditional knowledge, and, in some cases, the origin of the knowledge.
The figure below shows the distribution of 13,038 notifications of finished products registered in the system as of August 3, 2025. The data refers only to notifications where benefit-sharing is required and does not include exempted cases, which limit the ability to fully assess the weight of exemptions under Law no. 13,123/2015.
Figure 3. Distribution of Finished Product Notifications by Category
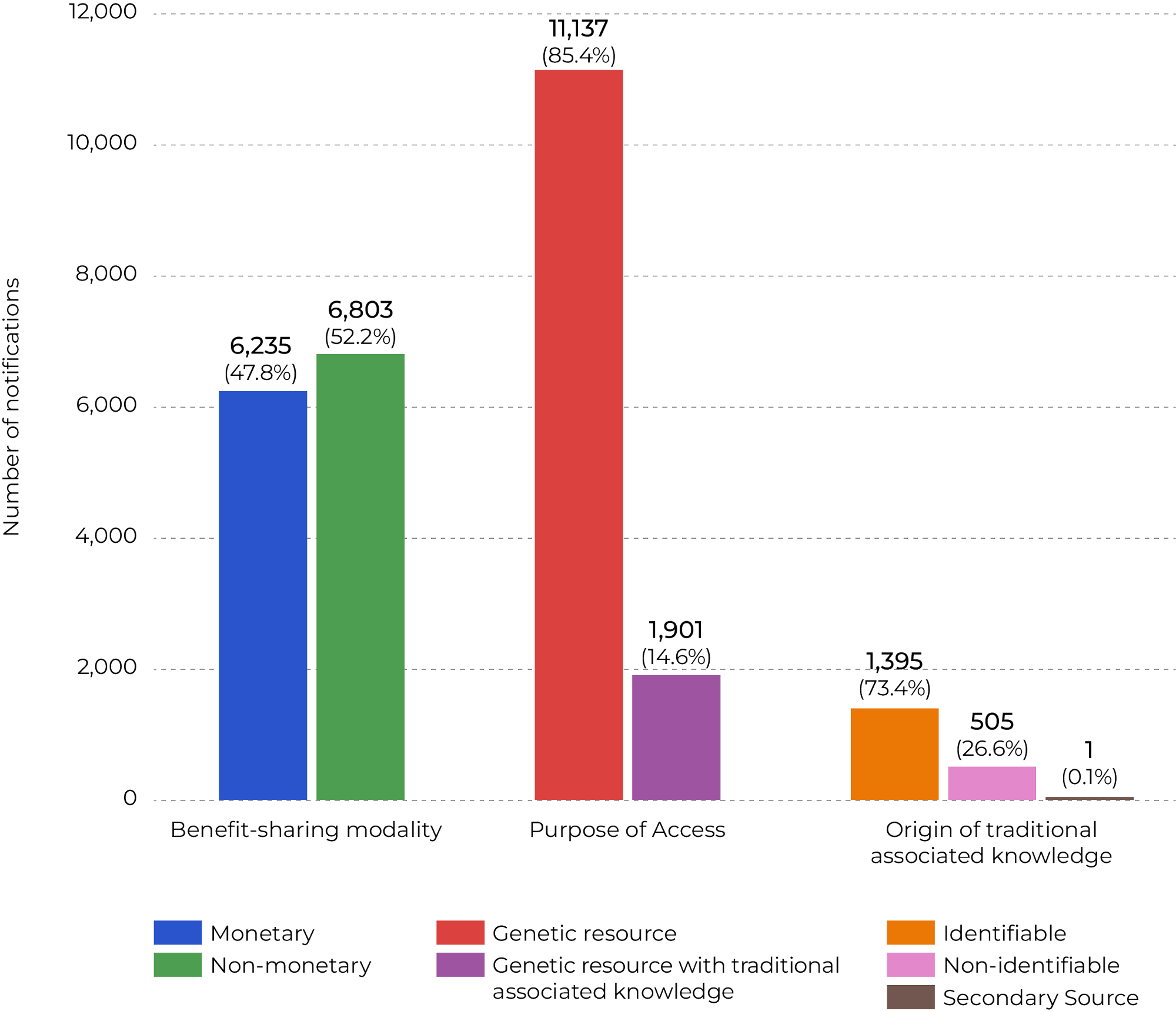
Source: CPI/PUC-RIO with data from SISGEN (2025), 2025
Based on the information recorded in the fields for biome, state, or provider community, 3,305 notifications, approximately 25% of the total, are linked to the Legal Amazon.[3] The next figure shows the distribution of these Amazon-related notifications by category, using the same structure as Figure 3.
Figure 4. Notifications of Finished Products Linked to the Legal Amazon (excluding Tocantins)
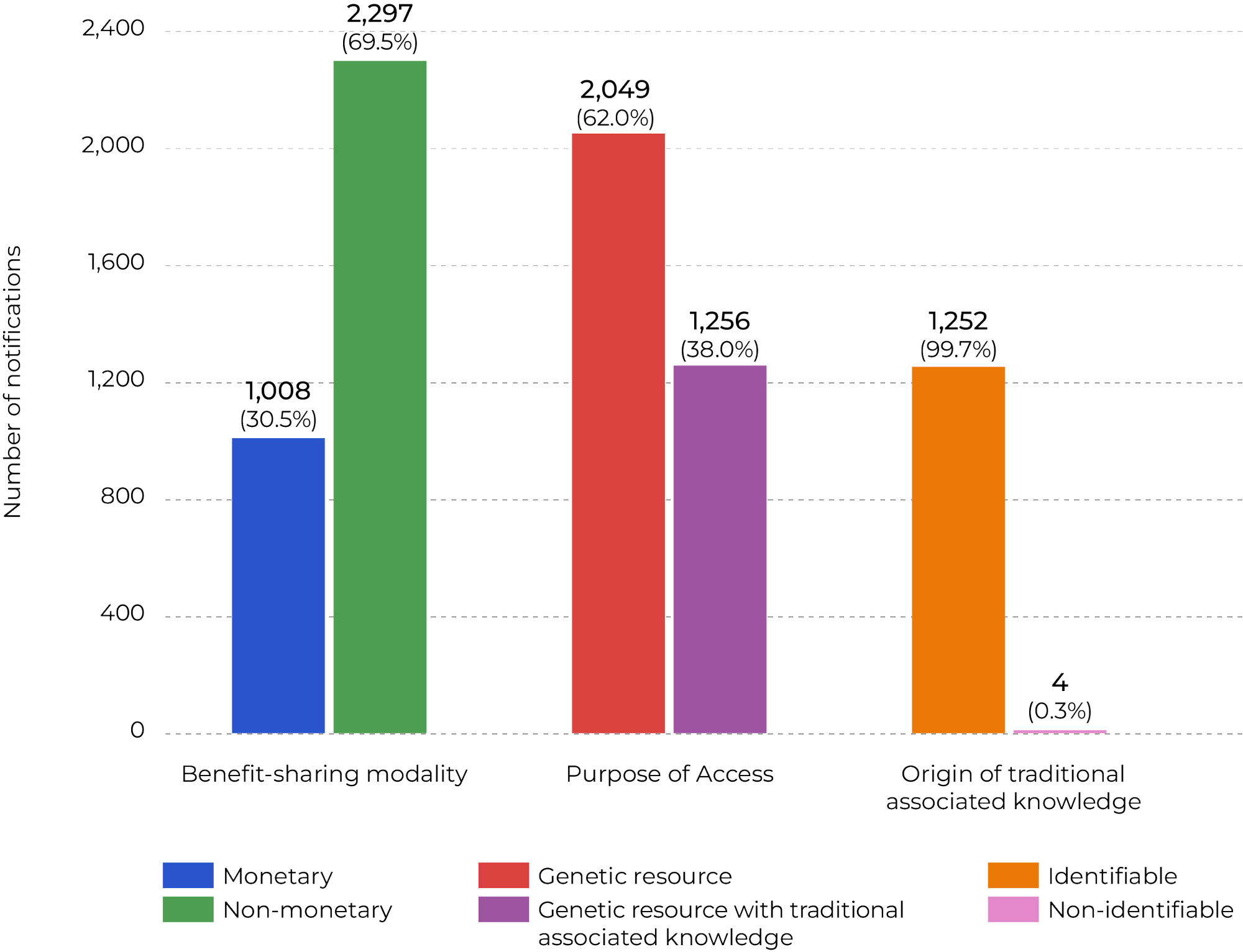
Note: Half of the notifications (50%) lack territorial information such as biome, state, or municipality, which limits traceability of the origin of genetic resources or associated traditional knowledge. This is partly because the origin of the sample may be an intermediate product.
Source: CPI/PUC-RIO with data from SISGEN (2025), 2025
While benefit-sharing in Brazil overall is relatively balanced between monetary (47.8%) and non-monetary (52.2%) modalities, in the Amazon, non-monetary benefit-sharing is predominant (69.5%). Access involving associated traditional knowledge is also proportionally more significant in the region, representing 38% of notifications compared to only 14.6% at the national level. Another key difference lies in the origin of traditional knowledge: in the Amazon, nearly all cases (99.7%) are of identifiable origin, which requires direct benefit-sharing with local communities, whereas at the national level, non-identifiable origin is more frequent (26.6%). These results indicate that, in the Amazon, biodiversity use is more strongly connected to traditional communities and non-monetary benefit-sharing, in contrast to the national profile, which is more centered on genetic resources (85.4%) and exhibits a greater diversity of benefit-sharing modalities.
SISGEN data reveal an important contradiction: although it is often argued that the benefit-sharing mechanism does not reach IPLCs (Uma Concertação pela Amazônia 2024; Instituto Escolhas 2023), in the Amazon a significant share of biodiversity-derived products involves traditional knowledge of identifiable origin, requiring direct benefit-sharing with local communities. In other words, where biodiversity and these communities are most present, the mechanism proves more effective. Even so, the ABS regime does not result in broad and predictable resource generation for conservation and for these communities, due to legal exemptions, the concentration of obligations in a few industrial sectors, CGEN’s difficulties in approving agreements, and the limited implementation of the FNRB.
Non-Monetary Benefit-Sharing Agreements with the Federal Government
Manufacturers of finished products that involve only genetic resources, without the use of associated traditional knowledge, may choose to enter into a non-monetary benefit-sharing agreement with the federal government. Under such agreements, users commit to developing projects or activities that benefit, for example, IPLCs or support biodiversity conservation.
The MMA has signed and approved 13 benefit-sharing agreements involving seven companies between April 2022 and December 2023 (MMA 2024a). Since then, no new agreements have been approved. Until approval is officially granted, users lack legal certainty to proceed, as investments already made cannot be counted toward benefit-sharing obligations if the agreement is rejected. These delays have been cited as one of the main obstacles to compliance (Instituto Escolhas 2021).
In addition to these delays, CGEN has been considering whether to revise pending agreements submitted by companies. A proposal under discussion would give the National Secretariat for Bioeconomy within the MMA the authority to verify—even in cases declared as involving only genetic resources—whether associated traditional knowledge was also used, by assuming that all access to genetic resources is inherently tied to traditional knowledge. In this scenario, agreements could be rejected, forcing users to negotiate benefit-sharing agreements directly with IPLCs, or traditional farmers holding such knowledge (MMA 2025b; MMA 2025c). This possibility creates significant legal uncertainty for users and may particularly affect the cosmetics, personal care, and perfume industry, which accounts for most non-monetary benefit-sharing in the country.
The recent adoption of CGEN Resolution no. 47/2025 adds new complexity to this debate. The resolution establishes reference lists of associated traditional knowledge, both identifiable and non-identifiable, which could be used by the National Secretariat of Bioeconomy to challenge cases initially declared as involving only genetic resources, including agreements still awaiting approval. However, Law no. 13,123/2015 does not recognize traditional knowledge as automatically inherent to genetic resources. Any new regulation should therefore apply only to future cases. Using these lists to revise agreements already submitted would further reinforce legal uncertainty.
National Benefit-Sharing Fund
The FNRB was created to redistribute monetary benefit-sharing contributions toward biodiversity conservation and support for IPLCs. From 2020 to February 2025, the Fund collected approximately R$ 9.9 million from activities involving access to genetic resources and associated traditional knowledge (MMA 2025d). This amount is minimal compared to the expectations placed on the framework.
Although the Fund has an Operations Manual and a Quadrennial Plan (2024–2027) with public guidelines for resource allocation, it remained inactive for several years and only made its first disbursements in June 2025. These were carried out through the first edition of the Guardians of Sociobiodiversity Award, designed to recognize representative organizations of traditional knowledge holders, including Indigenous Peoples, quilombola communities, family farmers, and other local communities (MMA 2025e). In August 2025, the MMA announced a second round of the award, but there are still no plans to allocate funds for other purposes (MMA 2025f).
While this represents some progress, the Fund’s execution remains slow and limited. As a result, the FNRB has not yet fulfilled its redistributive and socio-environmental role, falling short of its potential to generate continuous and structural impacts for communities holding traditional knowledge.
Access and Benefit-Sharing by Foreign Companies
Brazil’s ABS legislation applies equally to national and foreign users. Foreign companies, however, are required to indicate a Brazilian institution with which they have, or intend to establish, cooperation or an administrative association. This institution acts as a point of contact in the national territory. While intended to ensure a formal link to Brazil, this requirement has been cited as a barrier, particularly for smaller companies and research institutions that do not have established partnerships in the country.
Despite being a Party to the Nagoia Protocol, Brazil does not use the Protocol’s international Access and Benefit-Sharing Clearing-House as a channel for compliance verification. Instead, verification depends entirely on SISGEN, which operates only in Portuguese and remains difficult for foreign institutions to navigate. As a result, companies outside Brazil face added obstacles when trying to demonstrate compliance with Brazilian law.
Shipment of Genetic Resources Abroad
When a Brazilian research institution or company intends to ship a genetic resource sample abroad for research, product development, or deposit in a collection or database, it must, in addition to registering the shipment in SISGEN, sign an MTA with the foreign institution. The MTA functions as a contract that defines responsibilities: it makes the foreign institution legally responsible for the sample and alerts it to the application of sanctions in case of non-compliance with Law no. 13,123/2015. The MTA requirement can delay research or lead to refusal by the recipient. This affects the deposit of samples, the registration and publication of new species in collections and databases, and slows down the development of products abroad using Brazilian genetic resources, reducing partnerships and opportunities (da Silva et al. 2022).
Digital Sequence Information (DSI)
DSI refers to the use of genetic data in digital format, such as deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein sequences, which can be accessed without the need for a physical sample, through databases. Once deposited, this information becomes freely available to the public (Maia and Bourgeois-Gironde 2025). Because many sequences can be used to produce a single product, it is impractical to trace each sequence back to its country of origin for case-by-case benefit-sharing negotiations.[4] For this reason, the Parties to the CBD are developing a multilateral arrangement for benefit-sharing regarding DSI.
The proposal seeks to maintain open access to DSI databases but requires companies that benefit from these data, particularly in the pharmaceutical, nutraceutical, cosmetics, plant and animal breeding, and biotechnology sectors, to contribute to the newly created Cali Fund. For now, this proposal is non-binding, meaning that Parties may choose whether to adopt it, and contributions to the Fund remain voluntary (CBD 2024).
The central issue is how to align the Brazilian framework, which requires traceability of genetic information (including access, registration, and potential benefit-sharing), with the multilateral regime, in which access is expected to remain free, and benefit-sharing will be concentrated in the Cali Fund. Harmonizing national rules with this multilateral arrangement is essential to avoid regulatory fragmentation and ensure legal certainty. As DSI becomes increasingly important for R&D activities in biotechnology that rely on genetic sequences, continued legal uncertainty between Brazilian legislation and the mechanisms under development within the CBD may negatively affect the development of Brazilian biodiversity-based biotechnology.
Beyond the challenges stemming from the ABS framework, biodiversity-based biotechnology also faces obstacles related to financing and infrastructure (Box 2).
Box 2. Financing and Infrastructure Challenges
The development of biotechnology in Brazil still faces significant barriers related to financing, infrastructure, and coordination between research institutions and companies. Although the agriculture technology (agritech) sector has grown in recent years with increasing investments in biologicals and sustainable solutions for agribusiness, other biotechnology segments, such as health, cosmetics, and industrial applications, face greater difficulties in accessing capital that is compatible with long and high-risk technological cycles (Abstartups, 2024). Recent studies indicate that, in 2024, Brazil invested approximately R$ 1,16 billion in 41 startups across the agribusiness, food, and climate sectors, with an emphasis on biological and regenerative solutions, primarily concentrated in the Southeast (Peruchi 2025). By contrast, startups focused on health, pharmaceuticals, and cosmetics report more limited access to specialized investors (Mastellaro et al. 2024).
Public calls for proposals remain a relevant source of innovation funding, but they often include restrictive criteria, such as requiring researchers to be based in the project’s target region (SEBRAE and CONFAP 2023). This condition has been identified as a barrier to forming interregional partnerships and mobilizing complementary technical expertise, particularly in areas such as Amazonian biodiversity.
From an infrastructure perspective, there has been an expansion of centers and programs dedicated to biotechnology in different regions of Brazil. Despite some progress, coordination among universities, companies, and technology centers remains limited, with a concentration in the Southeast and South regions (Abstartups 2024).
There are also limitations in assessing the flow of resources allocated to research, development, and innovation, since there is not always transparency regarding the destination of these investments.
Impacts of Regulatory, Institutional, and Structural Barriers in the Amazon
The Amazon holds the greatest biological diversity on the planet and, at the same time, faces enormous challenges in transforming this potential into high-value-added technological solutions.
Legal requirements, such as the need to sign an MTA for the shipment abroad of genetic resource samples or their deposit in collections and databases, can affect the discovery and validation of new Amazonian species. Much of the region’s biodiversity remains unknown (National Geographic nd), and taxonomic classification often depends on shipping samples abroad for genetic sequencing. By creating a barrier to this process, Brazilian regulation restricts scientific research and the generation of knowledge about the Amazon and its potential for the development of new products.
When Law no. 13,123/2015 was enacted, there was an expectation that benefit-sharing would become a significant source of funding for the Amazon, supporting IPLCs and forest conservation. In practice, this promise has not materialized (Maia and Bourgeois-Gironde 2025; Euler, Aubertin and Cialdella 2023): legal exemptions reduce the scope of benefit-sharing, most agreements remain unapproved, and the FNRB has distributed only minimal resources. Added to this are the bureaucratic hurdles of SISGEN, which represent a central obstacle, especially for Amazonian startups and small businesses that lack the structure to cope with the system’s complex requirements.
The recent CGEN resolution on reference lists of associated traditional knowledge worsens this scenario. Since most Amazonian entries have been registered only as genetic resources, the presumption of inherently linked traditional knowledge may create new barriers, leading to increased bureaucracy, regulatory uncertainty, and a greater need for prior informed consent. This set of uncertainties amplifies the risk of deterring investment and encouraging jurisdiction shopping.[5]
The Amazon also suffers from weak coordination among government, research centers, companies, and local communities, which keeps efforts fragmented. The scarcity of public and private financing limits the predictability and scale of projects in the region (Uma Concertação pela Amazônia 2024). In addition, funding policies that require projects to be based in the region, although relevant to strengthening local capacity, can restrict the formation of strategic partnerships with institutions from other parts of the country (Kume 2023). This situation is exacerbated by broader structural challenges, including logistical bottlenecks, inadequate transportation, digital connectivity failures, and energy instability, which increase costs and reduce the competitiveness of biotechnology initiatives in the region (Ivarsson and Sekerinska, 2025; Veras, 2025).
Taken together, these challenges mean that the development of biodiversity-based biotechnology in the Amazon advances in a fragmented way, with difficulties in transforming the region’s scientific and biological potential into concrete solutions. The absence of a clear and stable regulatory environment, combined with infrastructure bottlenecks, limits the region’s capacity to attract strategic investment. Although biodiversity-based biotechnology has the potential to add value to sociobiodiversity chains and diversify the Amazonian economy, the Brazilian ABS regime alone will not generate the resources needed to finance this process.
PNDBIO as a Strategic Opportunity to Include Biodiversity-Based Biotechnology in the National Agenda
The National Bioeconomy Strategy, established by Decree no. 12,044/2024, aims to coordinate and implement policies and investments for the development of bioeconomy in harmony with civil society and the private sector. Its governance body is the CNBIO, composed of 15 ministries and representatives from other federal agencies, the private sector, academia, IPLCs, and the financial sector. CNBIO’s mandate is to propose guidelines, coordinate stakeholders, monitor actions, and oversee the preparation of PNDBIO, the key instrument for implementing the strategy.
The strategy defines bioeconomy as “a production and economic development model based on values of justice, ethics, and inclusion, capable of generating products, processes, and services efficiently, based on the sustainable use, regeneration, and conservation of biodiversity, guided by scientific and traditional knowledge, innovation, and technology, with the goal of adding value, creating jobs and income, ensuring sustainability, and supporting climate balance.”[6] Although centered around an ecological vision, this definition also acknowledges the role of innovation and technology in adding value and promoting sustainable development.
Despite this potential, the strategy does not explicitly mention biotechnology, instead focusing on a broader promotion of research, development, and innovation. The absence of explicit reference in the strategy to biotechnology restricts its recognition as a strategic dimension and may hinder its full incorporation into PNDBIO. The plan, currently under development, is structured around three components: i) bioindustry and biomanufacturing; ii) biomass; and iii) terrestrial and aquatic ecosystems and sociobioeconomy. The third component has already undergone public consultation, while the others are still in progress. According to the draft under consultation, the plan will be developed using the Mission-Oriented Policies methodology (MDIC, MF and MMA 2025).
The document makes broad reference to biotechnology in its conceptual framework and in identifying development opportunities, highlighting, for example, new materials, pharmaceuticals, enzymes, and functional ingredients derived from Brazilian genetic resources. However, this diagnosis is not reflected in the seven proposed missions: the topic appears only in the fourth mission, linked to the processing of agricultural and cattle biomass. The absence of references to biodiversity-based biotechnology reveals a mismatch between the acknowledged potential and the plan’s strategic priorities. This gap is not merely semantic. Without the explicit recognition of biodiversity-based biotechnology, Brazil risks narrowing the bioeconomy agenda to biomass and bioenergy, failing to fully explore the potential of its biological diversity.
In addition, the draft under public consultation also recognizes that the ABS framework constitutes one of the main regulatory barriers to advancing Brazil’s bioeconomy. As a response, the draft proposes the creation of a Regulatory Affairs Working Group within the CNBIO. This body would seek to articulate regulatory proposals across ministries and sectors, reduce regulatory complexity, increase legal certainty, and promote greater alignment between regulatory frameworks and the demands of bioeconomy. This initiative is directly connected to the challenges identified in this report, which highlights the ABS regime as one of the primary obstacles to the development of biodiversity-based biotechnology.
In this sense, the development of PNDBIO represents an opportunity to consolidate biodiversity-based biotechnology as a transversal pillar of Brazil’s bioeconomy, direct programs and investments based on biodiversity, while also addressing regulatory barriers through the Regulatory Affairs Working Group. For this contribution to be effective, PNDBIO must reflect the diversity of perspectives represented in CNBIO and translate this plurality into balanced missions and targets. In doing so, the plan could consolidate a shared understanding of bioeconomy in Brazil and ensure that biodiversity is effectively incorporated as a strategic pillar.
Conclusion
Biodiversity-based biotechnology holds significant potential for Brazil, particularly in the Amazon. It can diversify the economy, open new markets, and generate jobs, contributing to both forest conservation and the productive inclusion of local communities. This potential, however, is hindered by numerous challenges, especially regulatory and institutional barriers, that limit the transformation of biological wealth into innovation.
The ABS framework, designed to provide legal certainty and stimulate research, has become one of the main obstacles to the development of biodiversity-based biotechnology in Brazil. The complexity of SISGEN, uncertainties surrounding benefit-sharing agreements, the new regulation on reference lists of traditional knowledge, and the lack of clarity regarding digital genetic information create an unpredictable environment that discourages innovation and investment. These challenges affect a wide range of actors—from researchers and small businesses to biodiversity-intensive sectors, including cosmetics, personal care, and perfumery. Moreover, at the international level, additional requirements combined with Brazil’s absence from multilateral mechanisms increase compliance costs and foster jurisdiction shopping, further reducing the country’s attractiveness in global markets.
Beyond the ABS framework, Brazilian biodiversity-based biotechnology is subject to sectoral and science, technology, and innovation regulations. Although multiple frameworks apply, the ABS framework concentrates the greatest complexities and challenges in terms of governance, legal certainty, and implementation. This regulatory asymmetry is compounded by a fragmented institutional environment, in which different ministries pursue distinct visions of bioeconomy—from sociobiodiversity to biomass and bioindustry—without sufficient coordination to recognize the transversal role of biodiversity-based biotechnology. Although Brazil has a National Bioeconomy Strategy, the absence of explicit reference to biodiversity-based biotechnology highlights the risk that this agenda will remain invisible and a low priority.
In this context, the elaboration of PNDBIO is a strategic opportunity. More than aligning different ministerial perspectives, it requires explicitly recognizing biodiversity-based biotechnology as a central dimension of Brazil’s bioeconomy. By doing so, Brazil can transform a comparative advantage—its biological diversity—into a driver of sustainable and inclusive development, especially in strategic regions such as the Amazon.
References
Alavarsa-Cascales, Diego, María José A. González, Miguel Palma, Gerardo F. Baerbero and Ceferino Carrera. “Optimization of an Enzyme-Assisted Extraction Method for the Anthocyanins Present in Açaí (Euterpe oleracea Mart.) Agronomy 12, no. 10 (2022). bit.ly/4myH5ur.
Associação Brasileira de Bioinovação (ABBI). Identificação das Oportunidades e o Potencial do Impacto da Bioeconomia para a Descarbonização do Brasil: Novos Cenários. 2024. bit.ly/4n7UYPV.
Associação Brasileira da Indústria Química de Insumos (ABIFINA). Acheflan, primeiro medicamento 100 % brasileiro, será lançado no México. 2015. Access date: August 5, 2025. bit.ly/3VmYZE8.
Associação Brasileira da Indústria Química de Insumos (ABIFINA). Rede de Biodiversidade critica atraso no SisGen. 2017. Access date: August 5, 2025. bit.ly/47dU1Rr.
Associação Brasileira de Startups (Abstartups). Mapeamento do Ecossistema Brasileiro de Startups 2024. São Paulo, 2024. bit.ly/3Vt9Lc8.
Banco Nacional de Desenvolvimento Econômico e Social (BNDES). Nota Técnica AF/DEPOL nº 019/2024: Prestação de Contas FNRB. 2023. bit.ly/41vZHTl.
Bockmann, Flávio Alicino, Miguel Trefaut Rodrigues, Tiana Kohsldorf, Lorian Cobra Straker, Taran Grant et al. “Brazil’s government attacks biodiversity”. Science 360, no. 6,391 (2018): 865. bit.ly/3I11B7H.
Centro de Gestão e Estudos Estratégicos (CGEE). Oportunidades e Desafios da Bioeconomia. Perspectivas da Bioeconomia Brasileira com Base em Inovações Tecnológicas e de Mercado. Brasília: CGEE, 2020. bit.ly/3KEheif.
Chiavari, Joana, Miguel Motta, Cristina Lopes and Ana Flávia Corleto. Financiamento para a Bioeconomia no Brasil: Fontes e Destinação dos Recursos. Rio de Janeiro: Climate Policy Initiative, 2024. bit.ly/3HVEr2H.
Confederação Nacional da Indústria (CNI). Bioeconomia e a Indústria Brasileira. Brasília: CNI, 2020. bit.ly/3wGr1ik.
Convention on Biological Diversity (CBD). Decision adopted by the Conference of the Parties to the Convention on Biological Diversity: Digital sequence information on genetic resources. CBD/COP/DEC/16/2. 2024. bit.ly/3JCcTzS.
Corrêa, Alexandra B. de G. “Limites éticos a patentes biotecnológicas”. Revista Quaestio Iuris 13, no. 03 (2020): 1348–1374. bit.ly/4mEDKcZ.
da Silva, José Maria Cardoso, Luís Claudio F. Barbosa, Julie Topf, Ima Célia G. Vieira e Fabio R. Scarano. “Minimum costs to conserve 80 % of the Brazilian Amazon”. Perspectives in Ecology and Conservation 20 (2022): 216–222. bit.ly/47nwa1L.
Decree no. 12, 044, June 5, 2024. bit.ly/4mhN2uL.
Decree no. 8,772, May 11, 2016. bit.ly/4ndFvy0.
Euler, Ana Margarida C., Catherine Aubertin, and Nathalie Cialdella. “Amazon Socio-biodiversity in search of international markets.”. Estudos de Sociologia 28, no. 2 (2023). bit.ly/46p5iwl.
Farias, Talden, Bruna G. Maia and Paula S. Lima. “The Nagoia Protocol, Benefits from Genetic Resources and Brazilian Legislation.” Veredas do Direito 19, nº 43 (2022): 89-117. bit.ly/3HAQClu.
Feltran-Barbieri, Rafael, Juliana Brandão, Henrique Roncada, and Camila Barbosa. “Bioeconomia pode impulsionar crescimento e empregos na Amazônia”. WRI Brasil. 2025. Access date: August 6, 2025. bit.ly/3VlQw4m.
FIOCRUZ. Cartilha para a Academia. 2018. bit.ly/4g3b9vr.
Gibson, Keila. UFPA develops research for the production of biofuel and bioasphalt with açaí and tucumã seeds. 2024. Access date: August 5, 2025. bit.ly/4mAeaoR.
Georgallis, Panikos, João Albino-Pimentel, and Nina Kondratenko. “Jurisdiction shopping and foreign location choice: The role of market and nonmarket experience in the European solar energy industry”. Journal of International Business Studies 52 (2021): 853–877. bit.ly/4g4JcDv.
Gupta, Varsha, Manjistha Sengupta, Jaya Prakash, and Baishnab C Tripathy. Basic and Applied Aspects of Biotechnology. Singapura: Springer Singapore, 2017. bit.ly/4oWGwvP.
Hilgartner, S. “Biotechnology.” International Encyclopedia of Social and Behavioral Sciences, 2015. bit.ly/3VsB06B.
International Nucleotide Sequence Database Collaboration (INSDC). INDSC spatiotemporal metadata – minimum standards update (03-03-2023). 2023. Access date: August 6, 2025. bit.ly/48qYHnB.
Instituto Butantan. Por que é importante estudar os venenos dos animais? 2023. Access date: August 5, 2025. bit.ly/45LzBy4.
Instituto Escolhas. Monitoring the use of Traditional Knowledge: how can Brazil push this agenda? – Executive Summary. 2023. bit.ly/3VZKZAw.
Instituto Escolhas. Unlocking the Bioeconomy agenda: Solutions to boost the sustainable use of genetic resources and traditional knowledge in Brazil. São Paulo, 2021. bit.ly/4ncfgbz.
International Chamber of Commerce (ICC) Brasil. O potencial do Brasil na bioeconomia do conhecimento. São Paulo, 2025. bit.ly/4lNsJox.
Ivarsson, Ellin and Liljana Sekerinska. Uncovering Infrastructure Gaps in the Amazon: How to Leverage Data for Better Transport, Digital Connectivity, and Sustainable Development. Transport for Development – World Bank Blog, 2025. Access date: August 5, 2025. bit.ly/3JHcNqL.
Jungmann, Diana and Jorge de Paula Costa Avila. “Data-Driven Assessment on the Brazilian Regulatory Framework for Biodiversity Access and Benefit Sharing (ABS).” Preprint.org. (2022). bit.ly/4mSWBRG.
Kume, Wilson. “Análise de fatores para subsidiar modelagem de portal de suporte às startups de biotecnologia da Região Norte”. PhD Diss. in Biotechnology, Federal University of Amazonas, 2023. bit.ly/46gfqbM.
Law no. 13,123, May 20, 2015. bit.ly/3USd1xF.
Lopes, Cristina L. and Joana Chiavari. Bioeconomy in the Amazon: Conceptual, Regulatory and Institutional Analysis. Rio de Janeiro: Climate Policy Initiative, 2022. bit.ly/BioeconomyAmazon.
Machado, Pedro A. S. de Aguiar, Pedro Vitor de S. Silva, Matheus A. Fonseca, José Victor M. de Jesus, Naísa C. Leal et al. “Use of Macauba (Acrocomia aculeata) for Biofuels: Perspectives and Challenges in the Energy Sector”. Revista de Gestão Social e Ambiental 19, no. 6 (2025):1-12. bit.ly/4neDTnD.
Maia, Bruna G. and Sacha Bourgeois-Gironde. “Analysis of the Working Group’s Recommendations and COP-16 Decision 16/2 on Digital Sequence Information”. Global Policy (2025): 1-11. bit.ly/4lVrIea.
Malavazi, Fernando Wagner, Rodrigo D. Soliani, Genildo C. Ferreira-Júnior and Mario Sérgio P. Lobão. “Access to Genetic Resources and Associated Traditional Knowledge with Federal Science and Technology Institutions in Brazil: A Systematic Review of Regulatory Challenges”. Fronteiras: Journal of Social, Technological and Environmental Science 14, no. 1 (2025): 253-266. bit.ly/4lQwJ7J.
Mastellaro, Rosana, Isabela Allende, Marcio de Paula and Raquel Goes. Brazilian Healthcare Biotech Startups Guide. São Paulo: Sindicato da Indústria de Produtos Farmacêuticos (Sindusfarma), 2024. bit.ly/4p2EYQY.
Ministério do Desenvolvimento, Indústria, Comércio e Serviços (MDIC), Ministério da Fazenda (MF) e Ministério do Meio Ambiente and Mudança do Clima (MMA). Consulta Pública do Plano Nacional de Desenvolvimento da Bioeconomia: Documento-Base para Consulta Pública II. Brasília: MDIC, MF e MMA, 2025. bit.ly/461AiDI.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Resoluções. 2025a. Access date: August 5, 2025. bit.ly/3IymiIt.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Ata da 40ª Reunião Ordinária do Conselho de Gestão do Patrimônio Genético – CGen, realizada em 19 de março de 2025. Brasília: MMA, 2025b. bit.ly/3JHKus6.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Minuta de Resolução/“Diretrizes e Critérios para ARBNM com União” (CGen – Item 8.1). 2025c. bit.ly/461MjYu.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Demonstrativo de movimentação financeira do FNRB (28/02/2025). 2025d. bit.ly/4lSnbJm.
Ministério do Meio Ambiente e Mudança do Clima (MMA). MMA entrega Prêmio Guardiãs da Sociobiodiversidade para 20 organizações detentoras de conhecimentos tradicionais. 2025e. Access date: August 5, 2025. bit.ly/4p7nqDu.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Fundo de Repartição de Benefícios aumenta alcance e valor destinado ao Prêmio Guardiãs da Sociobiodiversidade. 2025f. Access date: August 5, 2025. bit.ly/3JAfGJZ.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Acordos de Repartição de Benefícios Não Monetária firmados. 2024a. Access date: August 5, 2025. bit.ly/4n4Zvm5.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Demonstrativo de movimentação financeira do FNRB (31/12/2024). 2024b. Access date: August 5, 2025. bit.ly/47Xqqfp.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Plano Quadrienal do FNRB (2024–2027). Brasília: MMA, 2023. bit.ly/3UXx0Lj.
Ministério do Meio Ambiente e Mudança do Clima (MMA). Manual de Operações do Fundo Nacional para a Repartição de Benefícios – FNRB. Brasília: MMA, 2022. bit.ly/4oXT1aJ.
Ministério do Meio Ambiente (MMA). Convenção sobre Diversidade Biológica. Brasília: MMA, 2000. bit.ly/3HZdhrx.
National Geographic Brasil. Amazônia é megabiodiversa. O quanto? Ninguém sabe. nd. Access date: August 5, 2025. bit.ly/3HVvSoy.
Peddle, Shawn D, Riley J. Hodgson, Ryan J Borrett, Stella Brachmann, Tarryn C. Davies et al. “Practical Applications of Soil Microbiota to Improve Ecosystem Restoration: Current Knowledge and Future Directions”. Biological Reviews of the Cambridge Philosophical Society 100, no. 1 (2025): 1–18. bit.ly/3JK5RJg.
Peruchi, Eduardo. “Agro e tech: o panorama de investimentos em agtechs no Brasil em 2024”. Blog Arara Seed. 2025. Access date: August 5, 2025. bit.ly/3VqLFi6.
Resolution CGen no. 28, August 25, 2021. bit.ly/3JFrLgY.
Resolution CGen no. 47, June 4, 2025. bit.ly/4oXZLFv.
Serviço Brasileiro de Apoio às Micro e Pequenas Empresas (SEBRAE) e Conselho Nacional das Fundações Estaduais de Amparo à Pesquisa (CONFAP). Edital SEBRAE/CONFAP nº 01/2023 – Programa Inova Amazônia: Módulo Tração. Brasília / Manaus: SEBRAE / CONFAP / FAPEAM, 2023. bit.ly/42azda1.
Souto Correa Advogados. SisGen opens test environment for foreign user module. 2025. Access date: August 5, 2025. bit.ly/3IjsX9u.
Stehlgens, Jéssica L., Weison L. da Silva and Amanda B. Carvalho. “Biocosméticos Amazônicos: A utilização do açaí (Euterpe oleracea Mart.) em estética e dermatologia”. Revista FT 29, no. 140 (2024). bit.ly/4n82Cd3.
The Economist. The world needs a better way to share genetic information. 2025. Access date: August 5, 2025. bit.ly/4mAtt1A.
The Nature Conservancy Brasil. Sociobioeconomia do Pará tem potencial para gerar mais de R$ 170 bilhões em renda em 2040. 2021. Access date: August 5, 2025. bit.ly/3JEuKWO.
Uma Concertação pela Amazônia, org. Ciência, Tecnologia e Inovação para as bioeconomias. São Paulo: Arapyaú, 2024. bit.ly/47OwiYn.
Uma Concertação pela Amazônia, org. Bioeconomia: a evolução do debate e repercussões nas Amazônias. São Paulo: Arapyaú, 2023. bit.ly/3VmIoR3.
Veras, Hemanuel. “Desafios da conectividade significativa na Amazônia”. Fundação Heinrich Böll Brasil. 2025. Access date: August 5, 2025. bit.ly/4gmOhaN.
This work is supported by a grant from Itaúsa Institute. This publication does not necessarily represent the view of our funders, partners and experts consulted.
The authors would like to thank Ana Paula Rodrigues Viana, Aryane Martins, Bruna Maia, Fabio Brasiliano, Fábio Silva Macedo, Julia Moreira Pupe, Juliana Nakayama, Luiza Helena da Matta Ribeiro, Manuela da Silva, Mario Augusto de Campos Cardoso, Patricia Mendes, Suikinai Nobre Santos, Tiago Giuliani, and Wladecir Oliveira for their reflections and shared knowledge, which enabled a broader understanding of the topic.
We would also like to thank Beto Veríssimo, Juliano Assunção, Salo Coslovsky, and Natalie Hoover for their comments and suggestions. In addition, we thank Giovanna de Miranda, Camila Calado, Alexandre Mansur, and Gustavo Nascimento for their work in reviewing and editing the text, and Julia Berry and Meyrele Nascimento for their graphic design work.
[1] For further details on Brazil’s ABS Legal Framework, see: Ministry of the Environment of Brasil (MMA), Secretariat of Biodiversity and Department of Genetic Heritage. Genetic Heritage, Associated Traditional Knowledge and Benefit-Sharing. 2022. bit.ly/4mlWP2E.
[2] Access the guide here: Ministry of Environment and Climate Change (MMA) and Department of Genetic Heritage. Practical Guide to testing the SISGEN ‘module for non-brazilian institutions’. 2022. bit.ly/3VxmElB.
[3] Notifications were included when the biome was classified as “Amazon” or when the location was within the Legal Amazon states (Acre, Amapá, Amazonas, Mato Grosso, Pará, Rondônia, Roraima, and Maranhão, with the exception of Tocantins, where Amazon forest cover is minimal). In states such as Maranhão and Mato Grosso, the available data does not allow for distinguishing whether access took place in the Amazon or Cerrado biome, which may result in a slight overestimation.
[4] As of May 2023, the International Nucleotide Sequence Database Collaboration (INSDC), which brings together GenBank, EMBL-EBI, and DDBJ, made it mandatory to include country-of-origin information in new deposits of genetic sequences. Before that date, providing this information was optional (INSDC 2023). In the 2024, the CBD COP16, held in Cali, reinforced this requirement by approving Decision 16/2, extending the obligation to other public databases of digital sequence information and consolidating the link between transparency, traceability of origin, and benefit-sharing (CBD 2024).
[5] “Jurisdiction shopping” is the practice of choosing, among different countries or legal systems, the one whose legislation is most favorable to a given interest. In other words, actors such as companies “shop” for the jurisdiction where the rules are more flexible or advantageous for their activities (Georgallis, Albino-Pimentel & Kondratenko 2020).
[6] Original text: “o modelo de desenvolvimento produtivo e econômico baseado em valores de justiça, ética e inclusão, capaz de gerar produtos, processos e serviços, de forma eficiente, com base no uso sustentável, na regeneração e na conservação da biodiversidade, norteado pelos conhecimentos científicos e tradicionais e pelas suas inovações e tecnologias, com vistas à agregação de valor, à geração de trabalho e renda, à sustentabilidade e ao equilíbrio climático”.